The use of oncolytic viruses for treatment of glioblastoma, an otherwise incurable brain cancer
Safe viruses that can cause lethal diseases in chicken, such as Newcastle disease virus (NDV), are sometimes used as oncolytic viruses (cancer killing viruses) to treat cancer safely. These pictures shown two patients with fully resistant recurrent glioblastoma, an otherwise incurable disease. One of the patients is currently 24 years ...
Read More

First Successful Gene Therapy for Infant with Severe Combined Immune Deficiency (SCID)
Severe Combined Immunodeficiency (SCID), stemming from Adenosine deaminase (ADA) enzyme deficiency, proves fatal due to inevitable infections, earning it the moniker “bubble baby” for the stringent isolation required to safeguard against contagion. The sole potential cure lies in successful gene therapy, wherein the ADA gene is introduced into multipotent stem ...
Read More

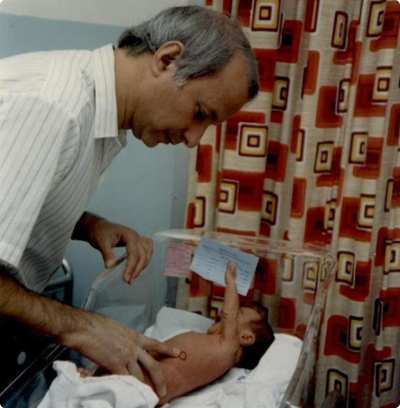
Intrauterine stem cell transplantation in preparation for treatment of genetic diseases
Many genetic diseases can be treated by stem cell transplantation from a normal donor. Many genetic disorders can be diagnosed during early pregnancy using a diagnostic amniocentesis or chorionic villus biopsy. Because of this, it was important to investigate if stem cell transplantation could be safely accomplished in utero. To ...
Read More
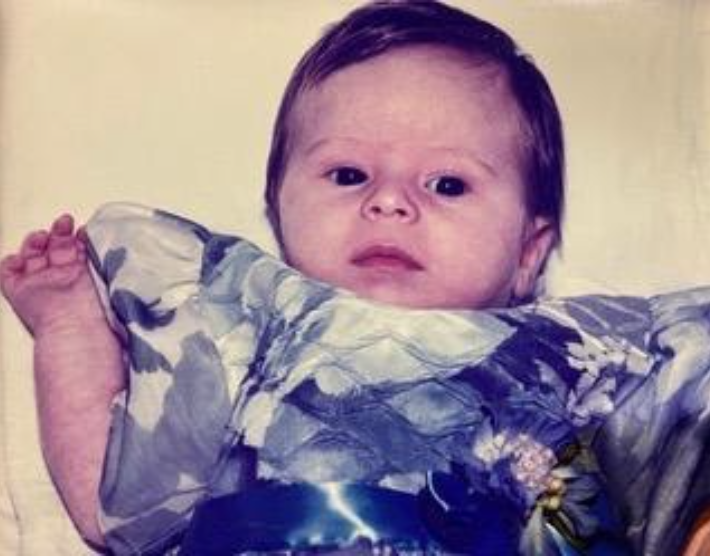
A baby with severe beta thalassemia major cured by allogeneic stem cell transplantation using reduced intensity conditioning
The patient shown above underwent allogeneic stem cell transplantation to treat severe beta thalassemia major. This is an otherwise incurable genetic blood disorder caused by an abnormal production of red blood cells, resulting from genetically-abnormal hematopoietic stem cells. Patients born with this disease depend on frequent blood transfusions. Eventually, many ...
Read More

1st successfully treated multi-transfused patient with severe aplastic anemia using reduced intensity non-myeloablative stem cell transplantation using total lymphoid irradiation (TLI) for prevention of graft rejection
This young woman presented with multiply-transfused severe aplastic anemia. Aplastic anemia is an acquired disease characterized by deficiency of hematopoietic stem cells. This results in severe anemia and a constant need of blood transfusions. Furthermore, the lack of production of white blood cells increases the risk of life-threatening infections. Poor ...
Read More

Using intentionally mismatched donor lymphocytes, including both T and NK cells, to induce stronger anti-cancer effects against minimal residual disease with no need for prior engraftment of donor’s stem cells
This patient, a 12-year-old girl from the Philippines, was referred to Prof. Slavin from UCLA for the potential treatment of acute myeloid leukemia, after conventional chemotherapy failed. No compatible donor was available and therefore, high dose myeloablative chemotherapy was accomplished, while the patient’s hematopoietic stem cells were cryopreserved ahead of ...
Read More
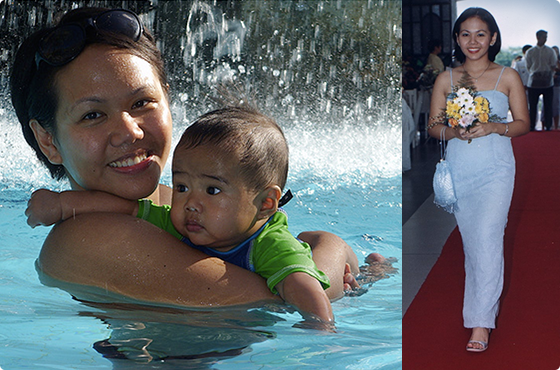
New Hope for a Cancer Cure: Using Mismatched Donor Lymphocytes
More than 20 years ago, a patient called Ece turned to Professor Shimon Slavin for treatment of an extremely aggressive blood cancer — acute myeloid leukemia — which was resistant to traditional chemotherapy. Ece had previously undergone a bone marrow transplant with high-dose chemotherapy, which was unsuccessful. Despite the professor’s ...
Read More

The first elderly patient who underwent successful allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia on an outpatient basis
Clinical application of NST or RIC instead of the conventional myeloablative transplant procedure made it possible to offer a cure for elderly patients with no upper age limit or to patients with poor performance status, using safer allogeneic stem cell transplantation procedure. Such patients would have been otherwise excluded from ...
Read More

The first patient that underwent successful allogeneic stem cell transplantation for advanced chronic myeloid leukemia using NST
The principle of NST was based on the idea that elimination of chemotherapy- and radiotherapy-resistant cancer cells could be accomplished using alloreactive donor lymphocytes post-grafting. This could also apply to a large number of hematopoietic stem cells producing Philadelphia-positive chronic myeloid leukemia (CML) cells. Such procedure should take into consideration ...
Read More

1st successfully treated patient with acute myeloid leukemia successfully treated using reduced intensity non-myeloablative stem cell transplantation
In early 1990, non-myeloablative stem cell transplantation (NST) or reduced intensity conditioning (RIC) in preparation for allogeneic stem cell transplantation, was introduced for the first time. The first successfully-treated patient was a young woman with acute myeloid leukemia. Treatment was uneventful and, following successful stem cell transplantation, the patient got ...
Read More