Using intentionally mismatched donor lymphocytes, including both T and NK cells, to induce stronger anti-cancer effects against minimal residual disease with no need for prior engraftment of donor’s stem cells
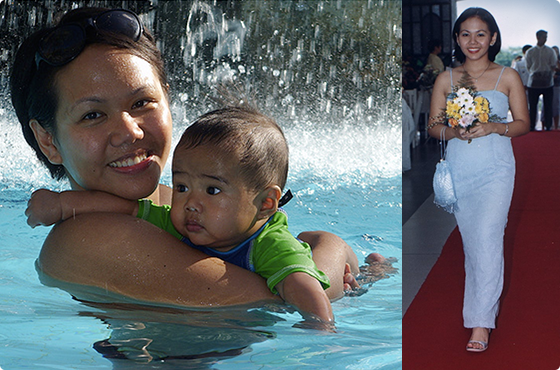
This patient, a 12-year-old girl from the Philippines, was referred to Prof. Slavin from UCLA for the potential treatment of acute myeloid leukemia, after conventional chemotherapy failed.
No compatible donor was available and therefore, high dose myeloablative chemotherapy was accomplished, while the patient’s hematopoietic stem cells were cryopreserved ahead of time. However, complete remission was not accomplished.
Therefore, based on prior successful experience using donor lymphocytes, Slavin treated her following autologous hematopoietic stem cell transplantation at the stage of minimal residual disease. He used haploidentical (mismatched) mother blood lymphocytes activated with interleukin 2 (IL-2), both before and after cell infusion. This was done to maximize the capacity of mismatched donor lymphocytes to kill otherwise resistant malignant cells.
The mother’s IL-2 activated blood lymphocytes, which contained both T cells and natural killer (NK) cells, were used for treatment under the assumption that no graft-vs-host disease (GVHD) will result because of anticipated rejection of donor’s T cells prior to development of GVHD.
Indeed, as predicted, the treatment was uneventful. After a successful course of cell therapy, she was for the first time free of any residual malignant cells. At present, 33 years later, she continues to present no signs of the disease, and has had no need for treatment since then. Our former patient is now a physician working in the US. She is the happily-married mother of 3 children.
The pictures shown below represents another example of anti-cancer effect of intentionally mismatched killer cells, including both T and NK cells, activated by IL-2 ex vivo prior to cell infusion and in vivo for 5 days following cell infusion in a patient with residual breast cancer liver metastases following high dose chemotherapy and autologous hematopoietic stem cell transplantation.
We have now documented that mismatched donor lymphocytes, activated with interleukin 2 (IL-2), can be most effective against chemotherapy-resistant cancer cells. Following this, more patients were treated in Slavin’s outpatient clinic after completing conventional chemotherapy procedures. For these patients, we used anti-cancer immunotherapy focusing on administration of IL-2-activated intentionally-mismatched donor lymphocytes. These were harvested family members or unrelated volunteers and some could even be cured.
Successful treatment with cell-mediated immunotherapy depends on treatment applied at the stage of minimal residual disease. Since mismatched donor lymphocytes are consistently rejected by the patient within a week, it is unlikely that treatment with intentionally mismatched killer cells could be effective in patients with late-stage bulky metastatic disease. Fortunately, a stage of minimal residual disease is normally accomplished in most patients with cancer, both in patients with hematologic malignancies and solid tumors. This is why it is important for patients and treating physicians to be aware of the potential benefits of anti-cancer immunotherapy at an early stage of the disease in patients at risk of recurrent disease.

